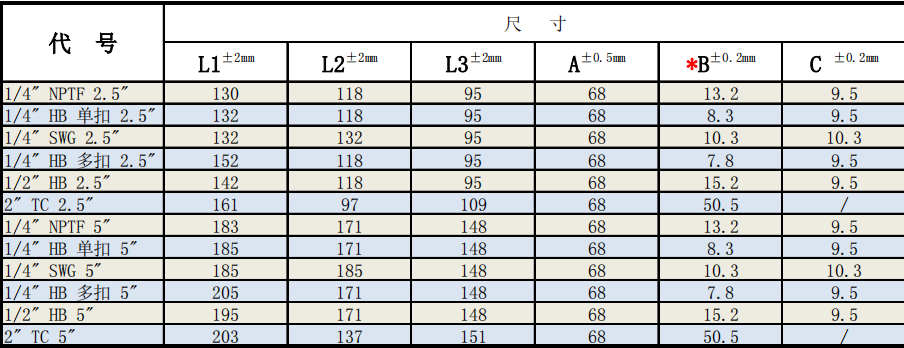
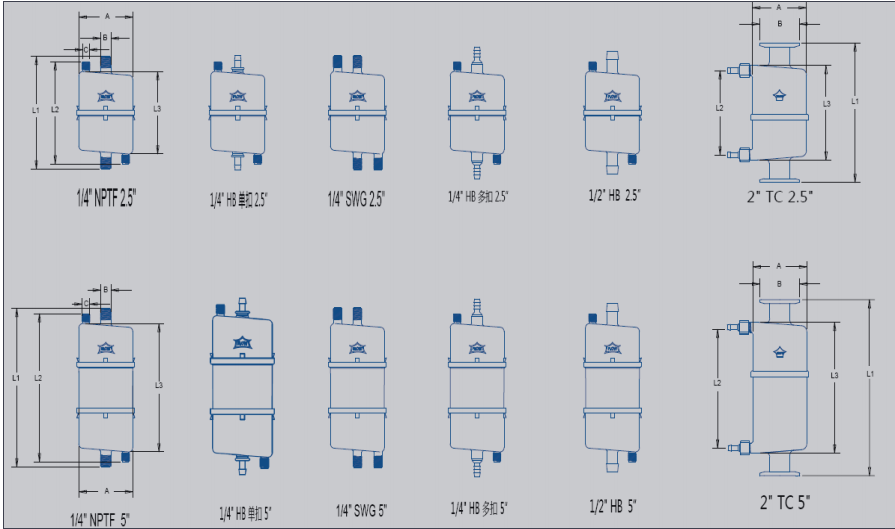
Sterilising-Grade PES Membranae Capsulae Filtrae
Description:
Filtra capsula puno PES familia est plenae magnitudinis capsulae filtrae
cum variis modis connexionis ad spiraculum, quod efficit in- tegrentiam rectam experiendi.
PES membrana reductionis capsulae bio-onus utilitas unius tabulati hydrophilici
membrana polyethersulfone.Lata praebet convenientiam chemica;
princeps fluxus rate et humilis extractables
Polyethersulfone aptissimum est ad liquationem productorum
continent substantias quae adsorb ad media inferiores notas obligandi
of polvethersulfone make it a good choice for filtration of valuable
dapibus solutiones ut vaccina et biologica
Beneficia
- C% integritas probata
- FDA cibum contactus obsequium
- Scelerisque bonding
- Non fibra releasing
Typicam Applicationem
- Cell Culture Media
- Magnae Volumen Parenterals (LVP's)
- Pharmaceutical Bulk
- Solutiones chemicas Diagnostics
- Sanguis et Serum Fractions
- Aqua mundata
- Beer, Vinum et Spiritus
- Succus & Mollis Bibentes
- Bottled aqua
Integritas Test Specifications
| Pore Size | 0.2um |
| Bulla Point | ≥1.4 barg(IPA/Aqua) |
| Aqua Intrusio | ≤0.37ml/min@ 2500mbar/10",22℃ |
| Fluxus diffusivus | ≤10ml/min @ 800mbar/10",22℃ |
Saccharum officinarum
2.5": 1400 cm²
5": 2500 cm²
10": 6000 cm²
20": 12000 cm²
Construction Materials
Membrana filtri: PES Membrane Media
Support: Polypropylene
Capsula: Polypropylene
Core interior: Polypropylene
Cavea exterior: Polypropylene
Obsignandi ratio: Scelerisque Bonding
Sanitization / sterilizatio
Autoclave circuitus 15 min @ 121 °C;non in linea vapor sterilizable